|
www.HealthyHearing.com |
Hearing loss in children
By Joy Victory, managing editor, Healthy Hearing  Reviewed by
Cher (Xue) Zhao, MD, pediatric otolaryngologist, Mass Eye and Ear Reviewed by
Cher (Xue) Zhao, MD, pediatric otolaryngologist, Mass Eye and Ear Last updated on: October 24th, 2022 Hearing loss in children can lead to speech and language delays. Learn more about the causes, symptoms and treatments available to children. If your baby or child has recently been diagnosed with hearing loss, you likely have a lot of questions and concerns. It's normal to feel overwhelmed and anxious, but rest assured that there are many treatment and adaptation options available, for both home and school. 
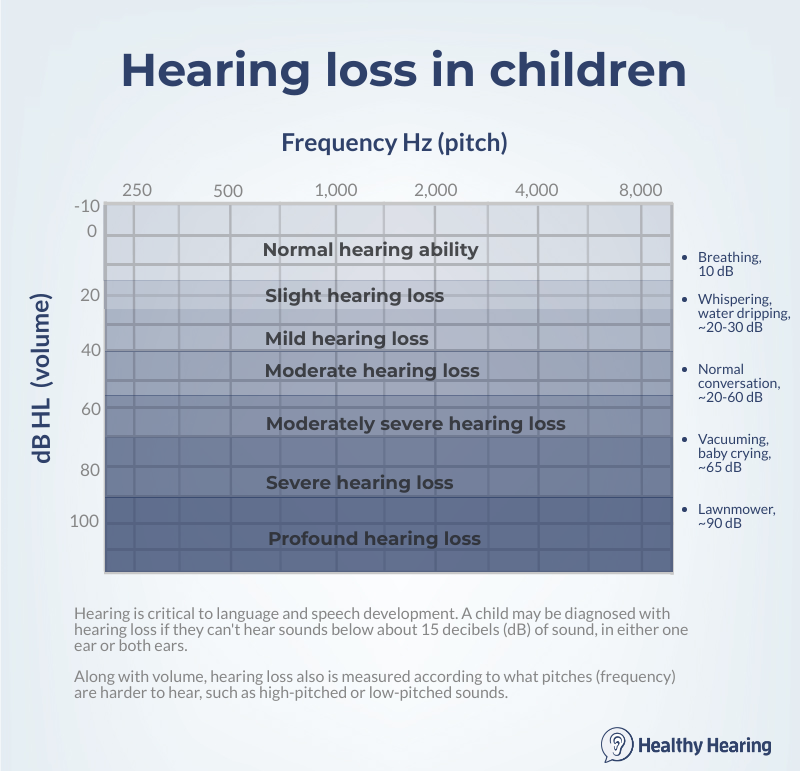
referred to an ear, nose and throat doctor and a pediatric audiologist. Perhaps the most important thing to know is that hearing ability helps a child develop her speech and language skills. It's critical to make sure your child's hearing loss is treated appropriately, to reduce the impact that hearing loss has on her education. In fact, research shows that treating hearing loss before a baby reaches six months of age results in significantly better speech and language outcomes than treating later. Research also shows that hearing aids boost school performance in children who have hearing loss. What is childhood hearing loss?A child may be diagnosed with hearing loss if they can't hear sounds below a certain level of volume, depending on the hearing test results, in either one ear (known as unilateral) or both ears (bilateral). The most minimal threshold is usually somewhere around 15 to 20 decibels (dB) of sound, which is roughly the sound of leaves rustling or people whispering. Even though being unable to hear leaves rustling would be considered a slight degree of hearing loss, it will make it harder to understand certain parts of speech. That's why treating hearing loss is so critical in kids, who are learning language from the moment they are born. That's a starting point, though. Some children will have mild hearing loss (they can't hear sounds below 25-40 dB), moderate (41-60 dB), severe (61-80 dB) and profound (81+ dB). Being unable to hear virtually all sound is known as profound deafness.
It's not just a matter of volumeHearing loss is a combination of loss of volume (measured in decibels) and loss of pitch, or frequency (measured in Hertz). For example, some children may struggle to hear sounds that are high-pitched, but have no problem hearing low-pitched sounds, known as high-frequency hearing loss. A child who has moderate hearing loss in the high frequencies will be unable to hear most speech correctly, but may hear a helicopter miles away with no problem. Some may have the reverse, which is low-frequency hearing loss, and others will have what's known as "flat" hearing loss, which means they struggle to hear sounds across all pitches, from low to high. As you can see, hearing loss is unique and highly variable, which is why customized hearing aids are extremely important, since they can be adjusted to a child's specific pattern of hearing loss. To fully understand your child's hearing loss, it's important to know the degree of hearing loss in each ear, as well as what pitches are harder for them to hear. Hearing loss at a young ageHow common is hearing loss in kids?There are many different estimates depending on the organization gathering the data, but overall hearing loss is fairly common in kids. One national survey estimated that about 15% of kids and teens have hearing loss, though in most cases the hearing loss was slight, and in only one ear. More severe levels of hearing loss are less common. More on hearing loss statistics. Signs and symptoms of hearing loss in babiesHospitals routinely perform newborn hearing screening on infants in the first day or two after birth. If a a newborn shows signs of infant hearing loss, he or she is usually scheduled for a second screening a few weeks later. However, sometimes newborns who pass both hearing screenings may exhibit signs of hearing loss as they get older.
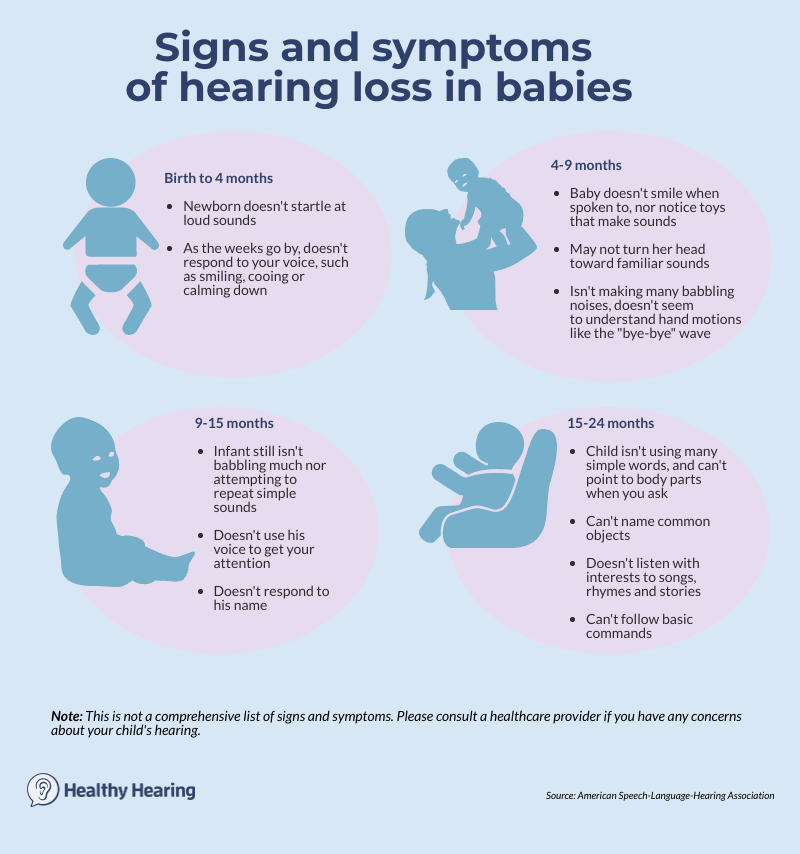
One way to determine if your child’s hearing is developing appropriately is by monitoring important speech and hearing milestones, such as these from the American Speech Language Hearing Association:
Babies and infantsFrom birth to four months, your infant should:
From four months to nine months, your infant should:
From nine to 15 months, your baby should:
From 15 to 24 months, your toddler should:
Signs of hearing loss in toddlers and school-age childrenOlder children sometimes develop hearing loss that wasn't present before. Here are some things to look for if you think your toddler or preschool-age child might have hearing loss:
More: What to do if you suspect your school-age child has hearing los What caused my child's hearing loss?When a child is born with hearing lossSome babies are born with hearing loss, which is known as congenital hearing loss. Many different things can cause this type of hearing loss, but it's not always possible to pinpoint the precise cause. In about half of all cases, the cause is genetic—meaning, inherited from a parent.
Genetic factors that might cause congenital hearing loss include:
Non-genetic factors that might cause congenital hearing loss include:
These non-genetic factors account for only around 25 percent of congenital hearing loss. For the remaining 25 percent, no cause can be found. When a child develops hearing loss laterChildren can also be affected by acquired hearing loss, meaning it occurs at any point after birth. There are various causes of acquired hearing loss, including:
Sometimes, the cause of the hearing loss is temporary, such as these four common causes of temporary hearing loss in kids. Auditory processing disorder in kids with normal hearingSome kids may pass traditional hearing tests, but still struggle to listen and understand speech, particularly in noisy environments. They may ask you to repeat what you've said a lot, and struggle to differentiate similar sounding words. If this sounds like your child, it's important to talk to your doctor, as it could be auditory processing disorder. This means the ears can detect sound adequately, but the brain struggles to interpret the sound correctly. Temporary hearing loss in childrenSome children may experience hearing loss that comes and goes, known as temporary, transient or fluctuating hearing loss. Even though it may not be lifelong, this kind of hearing loss is still harmful to speech and language development. Transient hearing loss can be caused by otitis media, more commonly known as a middle ear infection. One out of every 4 kids have had one episode of otitis media by the time they are three years old. This type of infection is very common in children because of the Eustachian tube position during childhood. The eustachian tube, which allows for air pressure equalization between the middle ear and the nasopharynx, is smaller and more horizontal during development. Thus, it is very susceptible to blockage by fluids or large adenoids (glands in the throat area). A middle ear infection can cause transient hearing loss because excess fluids in the keep the tiny middle ear bones from working properly. Thankfully, this type of hearing loss is usually temporary and resolves itself. However, frequent, untreated middle ear infections can cause cumulative damage to the bones, eardrum or auditory nerve, creating a permanent, sensorineural hearing loss. Hearing tests for childrenNewborn hearing screeningsNewborn hearing screening is mandated across all 50 states, requiring that newborn babies have their hearing screened before leaving the hospital. There are two types of newborn hearing screenings, both are painless and can be done while the baby sleeps. Read more about newborn hearing screening. Hearing testing for babies, toddlers and kidsInfant hearing testing: Visual reinforcement audiometryPediatric audiologists can test infants as young as six months of age behaviorally in a sound-treated booth using a test called visual reinforcement audiometry (VRA). VRA takes advantage of an infant’s reflexive head turn toward sound. In this test, a parent will hold their child on their lap while they sit on a chair in the center of the sound booth. The audiologist will play sounds or talk through speakers that are oriented to the left and right of the child. 
assess hearing in babies and toddlers. When the child hears the sound and looks toward it, he or she is rewarded with a visual reinforcement toy like a flashing light or dancing bear. The infant will usually stay engaged long enough for the audiologist to get a good indication of hearing ability for at least the better hearing ear. The visually-appealing toys lose their ability to hold the child’s attention once they are toddlers. Around the age of two, social praise will work for behavioral testing. Toddler hearing tests: Play audiometryA pediatric audiologist will use a method of testing called play audiometry for toddlers and preschool children. It's a hearing test that is made into a game for toddlers. The parent or assistant will sit on the floor in the booth with the child and train them to respond to any sound they hear by doing a certain task, like putting a block in a bucket. When the child correctly responds to a sound, the parent or assistant sitting with them will cheer with enthusiasm. Like the visual reinforcement described above, this age-appropriate social reinforcement will typically keep the child engaged long enough for the audiologist to get a good indication of hearing ability at least for the better hearing ear. If the toddler will wear earphones, ear-specific information can be obtained. Hearing tests for kidsOnce a child is school-aged, he or she can usually sit still, remain quiet and raise a hand in response to speech and tone stimuli in the sound booth. At this point, the child can easily wear headphones for ear-specific measurements and sit still for tympanometry and acoustic reflex tests, similar to adult hearing tests. Treatments for childhood hearing lossDepending on the severity and cause of hearing loss in your child, hearing aids, cochlear implants and a combination of speech therapy or assistive listening devices might be recommended forms of treatment. If a child has wax buildup, an ear infection or another problem causing temporary hearing loss, the ENT can treat it first to see if it helps with hearing loss.
Audiologists can perform in-depth behavioral hearing examinations for even very young children (as young as 6 months). There are several objective tests that infants, toddlers and young children can undergo as well. These tests are painless and non-invasive. After the exam, the audiologist will spend time talking with you about your child's hearing ability and recommend an appropriate treatment plan or medical intervention. Hearing aidsHearing aids are just one kind of device that can help children with hearing loss hear clearly again. There are many pediatric hearing aids, including high-powered aids for children with profound hearing loss that offer high-quality hearing assistance. Many solutions for children include special coverings and other accessories to ensure that young children don't remove or misplace their hearing aids. Cochlear implantsCochlear implants are surgically implanted devices that directly stimulate the auditory nerve in the inner ear with electrical stimulation. Cochlear implants also have an external device, and many companies make kid-friendly devices that can be held on with a soft headband. Cochlear implants work for infants and children who cannot benefit from hearing aids. Bone-anchored hearing systemsIn some cases, a child may be a better candidate for a bone-anchored hearing system. People who typically get the greatest benefit from bone-anchored hearing systems include those who have severe outer or middle ear malformations, such as microtia and atresia, and those with single-sided deafness. Speech therapyFor children who have had hearing loss that has affected their speech, he or she might need speech-language therapy after getting hearing aids or a cochlear implant to help him or her catch up on speech delays. Children with auditory processing disorder also can receive therapy to strengthen the way your children understands and uses language. Assistive listening devicesMany hearing aid manufacturing companies offer assistive listening devices such as FM systems that are discreet and work well in a classroom situation in conjunction with the child's hearing aid or cochlear implant. FM technology helps overcome the poor acoustics of classroom settings or other venues with lots of background noise. Essentially, the teacher wears or has a discreet microphone in front of him or her that transmits his or her voice directly to the child's hearing aids or cochlear implant, or to speakers around the classroom. Hearing loss prevention for youthYou can help your child have a lifetime of healthy hearing by protecting her hearing from loud sounds, including headphones. Noise-induced hearing loss is on the rise in kids and teens. Headphone use in particular is linked to early-onset hearing loss. School helpUntreated hearing loss negatively affects school performance. Parents aren’t the only ones with a responsibility to make sure certain children with hearing loss have an equal opportunity to learn; schools have responsibilities as well. Most children with hearing loss can be mainstreamed into standard classrooms. An educational audiologist can be an invaluable resource as you navigate your options. Finding a provider for your childSeek help right away if you suspect your child has hearing loss. Oftentimes, your pediatrician is a good place to start. She may refer you to a pediatric audiologist or an ear, nose and throat doctor. This primer on different types of hearing specialists may come in handy as you navigate your child's journey to better hearing. This article has been medically reviewed by physician Cher (Xue) Zhao, MD, a pediatric otolaryngologist at Mass Eye and Ear who sees patients at the Newton and Wellesley locations. Dr. Zhao earned her medical degree from the University of Michigan Medical School before completing her residency in otolaryngology–head and neck surgery there. She then completed her pediatric otolaryngology fellowship at Boston Children’s Hospital. Her clinic interests include pediatric hearing loss, head and neck masses, endoscopic ear surgery, salivary gland disorders, aerodigestive disorders, sleep surgery and sinus disease. Joy Victory, managing editor, Healthy Hearing
You are reading about: Related topics More information about hearing aids, hearing aid brands, assistive devices and tinnitus. Featured clinics near me
Earzlink Hearing Care - Reynoldsburg
HearingLife - Pickerington Find a clinicWe have more hearing clinic reviews than any other site! Related contentThe Healthy Hearing Report |
|
www.HealthyHearing.com |
Hearing loss in children
By Joy Victory, managing editor, Healthy Hearing  Reviewed by
Cher (Xue) Zhao, MD, pediatric otolaryngologist, Mass Eye and Ear Reviewed by
Cher (Xue) Zhao, MD, pediatric otolaryngologist, Mass Eye and Ear Last updated on: October 24th, 2022 Hearing loss in children can lead to speech and language delays. Learn more about the causes, symptoms and treatments available to children. |



 Joy Victory has extensive experience editing consumer health information. Her training in particular has focused on how to best communicate evidence-based medical guidelines and clinical trial results to the public. She strives to make health content accurate, accessible and engaging to the public.
Joy Victory has extensive experience editing consumer health information. Her training in particular has focused on how to best communicate evidence-based medical guidelines and clinical trial results to the public. She strives to make health content accurate, accessible and engaging to the public.